Computer-Aided Molecular Design of Ionic Liquids: Prediction of Melting Point
Derick C. Weis
derick.weis@monash.edu
School of Chemistry
Monash University
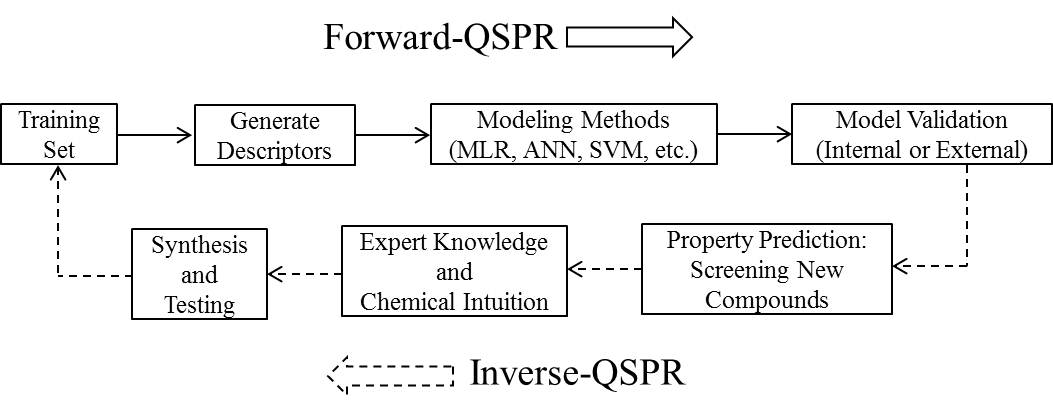
Ionic liquids (ILs) are salts that have melting points or glass transition temperatures below 100oC.[1] There are several features about this class of liquids that are attractive to researchers as solvents. Compared with conventional solvents, ILs typically have a broad liquid range with high thermal stability. Separation and recycling is possible by exploiting negligible vapor pressure. The properties of ILs can be modified by selection of the specific cation and anion pair; accordingly, applications have resulted in a wide variety of areas. One of the current challenges associated with designing novel ILs for specific applications is accurately estimating physical properties to save time and resources. Ideally, one would like to design new ILs on a computer predicted to possess the desired properties before synthesis and testing in the laboratory. Computer-aided molecular design (CAMD) is a technique well suited for such a task, and provides an excellent complement to the chemical intuition possessed by experimentalists.[2] Property predictions are obtained from a quantitative structure–property relationship (QSPR) that links changes at the molecular structure level to differences in the macroscopic properties. Given the numerous possible combinations of cations and anions available,[3] ILs offer an excellent opportunity for the application of CAMD to fine-tune physical properties. In this work, the general methodology for CAMD with QSPR (Figure 1) applied to predict the melting point for new ILs will be presented.

Figure 1. The general methodology for computer-aided molecular design (CAMD).
[1] An Introduction to Ionic Liquids (Ed. M. Freemantle) 2010, Royal Society of Chemistry: Cambridge, UK.
[2] Weis and MacFarlane, Aust. J. Chem. 2012, 65(11) 1478-1486.
[3] Katritzky et al., J. Chem. Inf. Comput. Sci. 2002, 42, 225.